Role of hypoxia and hif-1alpha in bone metastasis
Role of hypoxia and hif-1alpha in bone metastasis
Bone metastasis represents a frequent and severe complication of advanced breast cancers, and is associated with pathological fractures, intractable pain, and reduced quality of life. Thus, understanding the molecular and cellular mechanisms that contribute to metastasis to bone is crucial in order to find new therapeutic approaches to prevent these dreadful consequences. Most studies of breast cancer formation and metastasis have focused on microenvironmental factors within the primary mammary tumors, or genetic and epigenetic factors within the breast cancer cells that are responsible for their growth and dissemination. Our lab has undertaken a converse approach, focusing on one of the distant target tissues (bone) where breast cancer cells like to establish secondary tumors, rather than on the primary tumors. Our work aims to acquire a better understanding of how the bone microenvironment influences bone metastasis.
Since Stephen Paget’s “seed and soil” hypothesis from more than a century ago, it is known that bones constitute a very hospitable soil that attracts and allows breast cancer cells (the seeds) to thrive. Surprisingly, however, only a few studies have addressed the role of the bone microenvironment in bone metastasis, and most of them emphasized the role of osteoclasts (bone-destructing cells). The role of osteoblasts (bone-making cells) in this process is unknown. We tested the possibility that osteoblasts, like osteoclasts, stimulate bone metastasis.
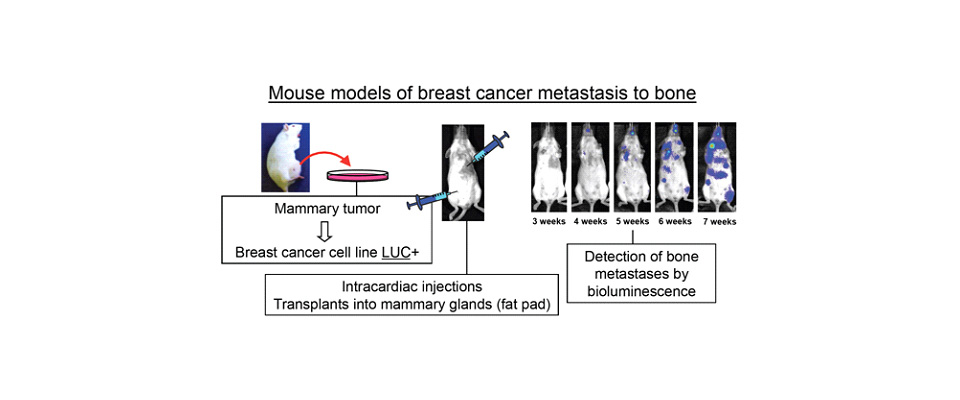
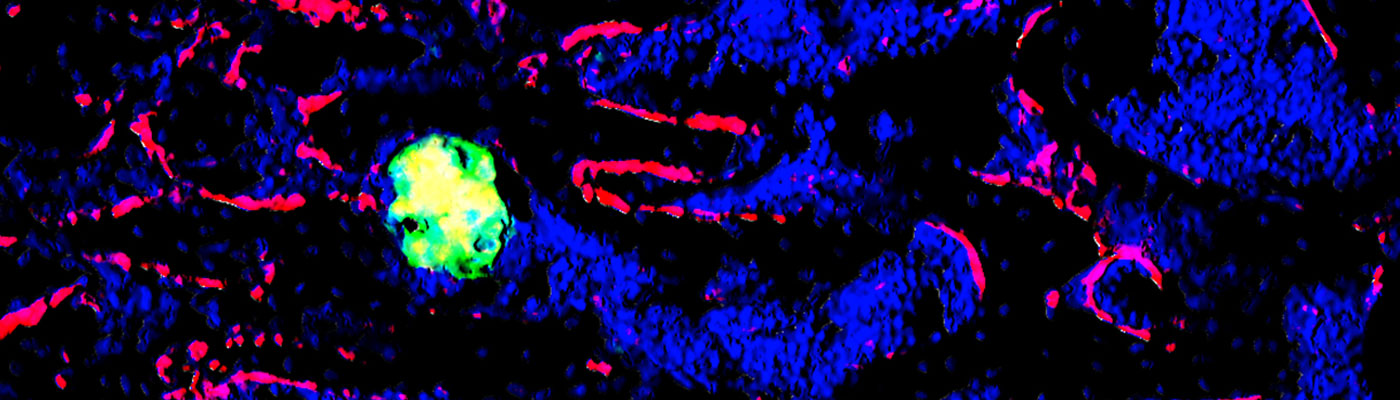
Hypoxia/Hif-1α plays a fundamental role in bone enhancing the differentiation of the osteoblasts, in hypoxic niches where metastatic cells coming form distant tumors are often found. However, the role of the hypoxic microenvironment in bone metastasis is unknown. Analyses of cell specific loss- or gain-of-function models for Hif-1alpha in mice revealed that osteoblasts directly or indirectly stimulate breast cancer metastasis to bone. These results were obtained by either intracardiac injections of mammary tumor cells, or orthotopic engraftment of these cells into the mammary fat pad of syngeneic recipient mice. Our current goal is to understand the cellular and molecular basis responsible for this phenomenon.