Role of hypoxia and hif-1alpha in skeletal development
Role of hypoxia and hif-1alpha in skeletal development
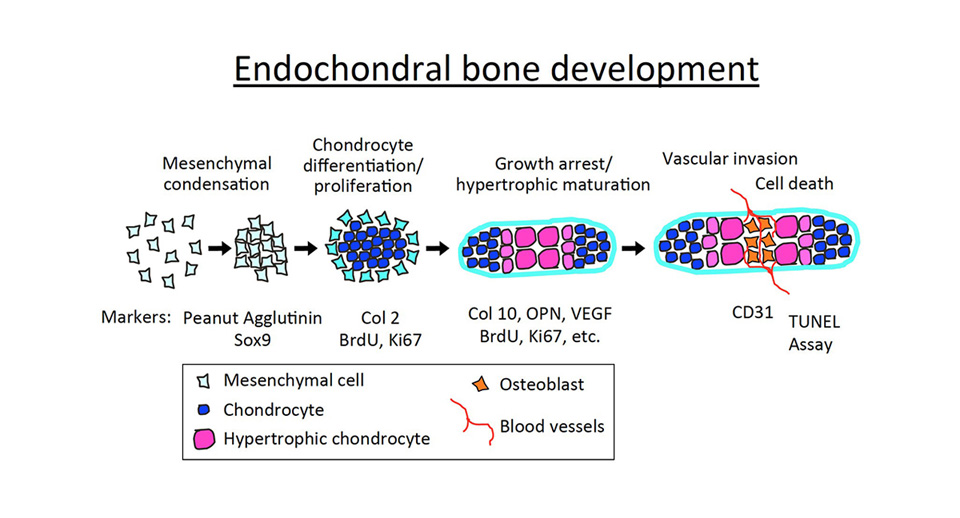
The human skeletal system consists of 206 bones. Most of them are formed through a process called endochondral bone formation, whereby mesenchymal cells differentiate into chondrocytes (the cartilage-making cells), establishing a cartilaginous matrix, which is then progressively replaced by bone. This process involves a multi-stage mechanism which starts early during embryonic development with the condensation of mesenchymal cells, which then differentiate into immature chondrocytes. Chondrocytes undergo well-ordered and controlled phases of cell proliferation, maturation (hypertrophic differentiation), and apoptosis, forming a cartilaginous template called the growth plate. Proliferating chondrocytes synthesize type II collagen, and then terminally differentiate into post-mitotic hypertrophic chondrocytes that express predominantly type X collagen (Col X). Hypertrophic chondrocytes eventually die off, and cartilage is progressively replaced by bone. Our work and that of others demonstrated that hypoxia and its main effector, the Hypoxia Inducible Factor-1alpha (Hif-1alpha play a critical role in multiple steps of skeletal development.
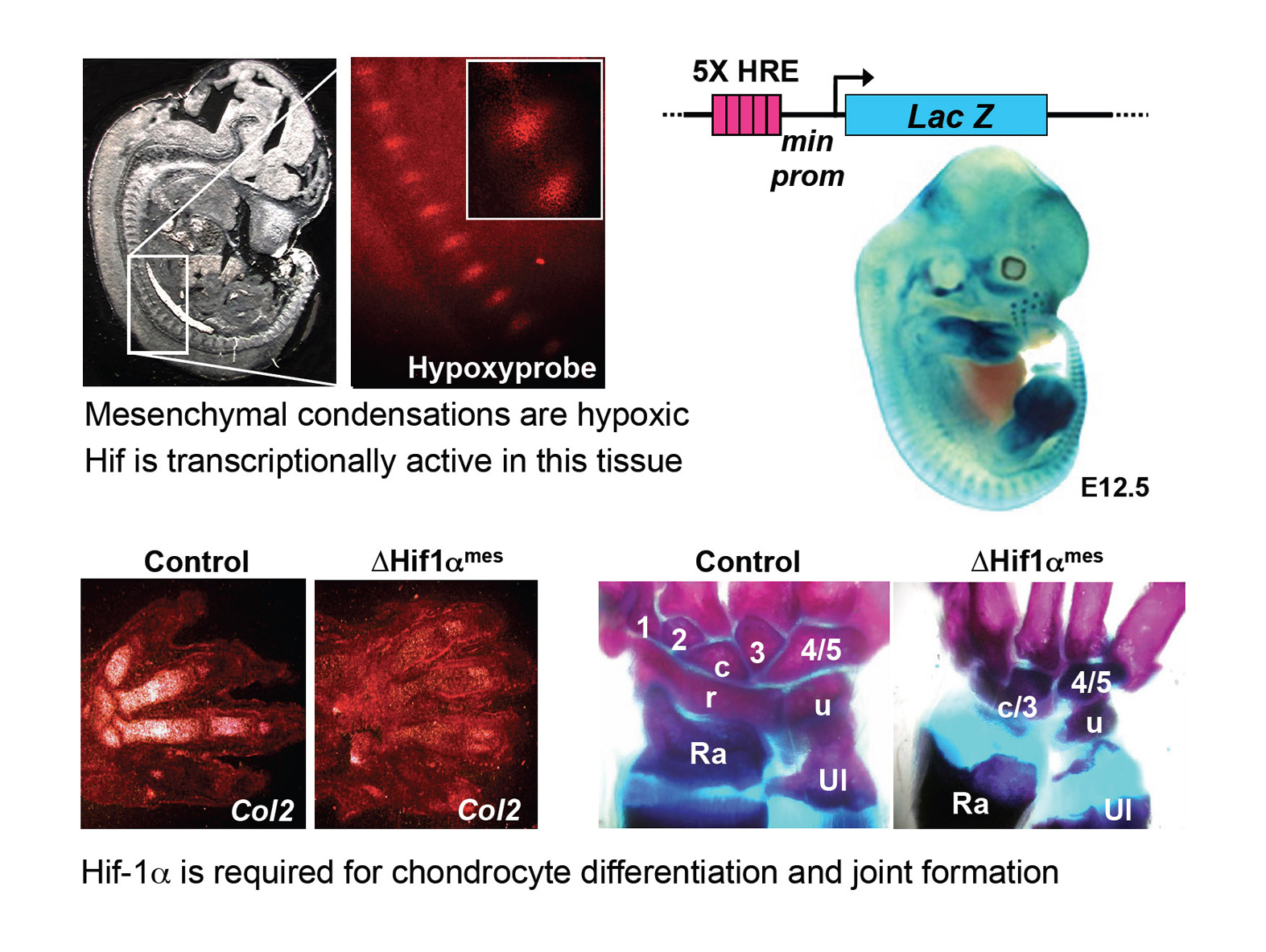
Hif-1alpha mediates the transcriptional responses to localized oxygen deprivation (hypoxia) in normal tissues and in cancers. This transcription factor is degraded in well-oxygenated conditions, whereas it is stabilized and activated in hypoxic conditions (≤5% O2). The von Hippel–Lindau tumor suppressor gene (VHL) is an E3 ubiquitin-ligase that targets Hif-1lpha for degradation by the proteasome, in non-hypoxic tissues. Genetic tools in mice allowed us to demonstrate that Hif-1alpha is required for the differentiation of mesenchymal cells (which became hypoxic upon mesenchymal condensation) into chondrocytes. Moreover, we established that Hif-1alpha is required in the mesenchyme for the development of joints, which also arise from condensed hypoxic mesenchymal cells. The cartilage is a unique tissue since it is avascular, and it requires an angiogenic switch to be replaced by bone. Hif-1alpha is necessary in cartilage to maintain chondrocyte survival, while it inhibits their proliferation. Moreover, this factor stimulates the deposition of cartilaginous extracellular matrix. Lastly, Hif-1alpha plays a crucial role in bone by promoting osteogenesis, possibly indirectly by inducing the expression of the Vascular Endothelial Growth Factor (VEGF, a well known direct target gene of Hif-1alpha) in osteoblasts, and stimulating angiogenesis.
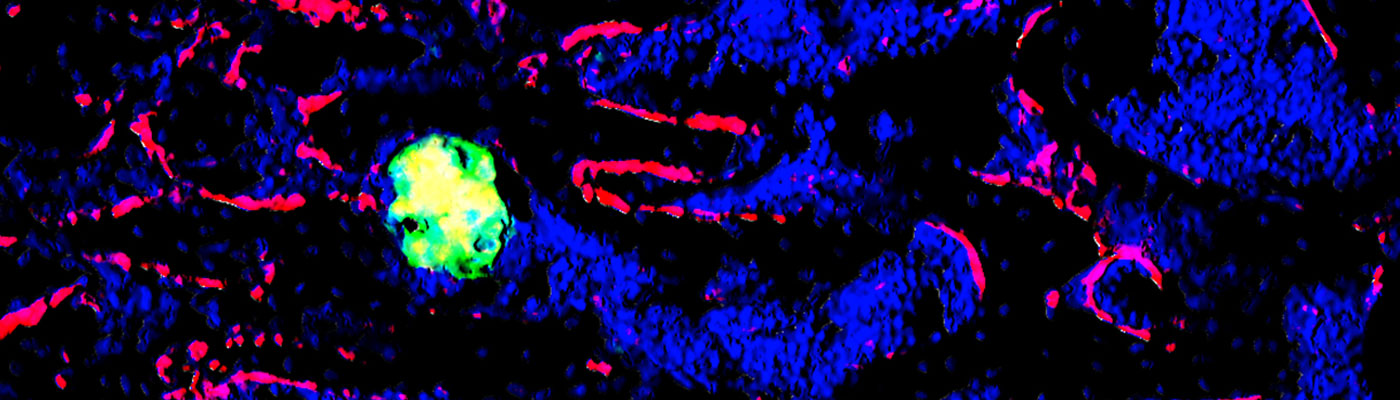
Hypertrophic differentiation of chondrocytes is a mandatory step in skeletal development. Accelerated, or conversely delayed hypertrophic differentiation of chondrocytes results in skeletal defects that can lead to death or severe dwarfisms. Hypertrophic chondrocytes are defined by the appearance of enlarged (hypertrophic) chondrocytes that express Col X, Osteopontin, VEGF, and other markers. Despite their importance, relatively little is known concerning the mechanisms controlling cellular hypertrophy in cartilage. We investigated the role of Hif-1alpha in cellular hypertrophy in cartilage. Or unpublished results demonstrate that oxygen homeostasis, and Hif-1alpha, are essential in this process. In particular, we observed that abnormal levels of hypoxia and HIf-1alpha protein are associated with abnormal hypertrophic differentiation of chondrocytes. Since multiple chondrodysplasias result at least in part from defective cellular hypertrophy, we would like to investigate the role of hypoxia/Hif-1alpa in pathological cartilage developments, and evaluate whether targeting Hif-1alpha could constitute a valid therapeutic approach in skeletal diseases.